
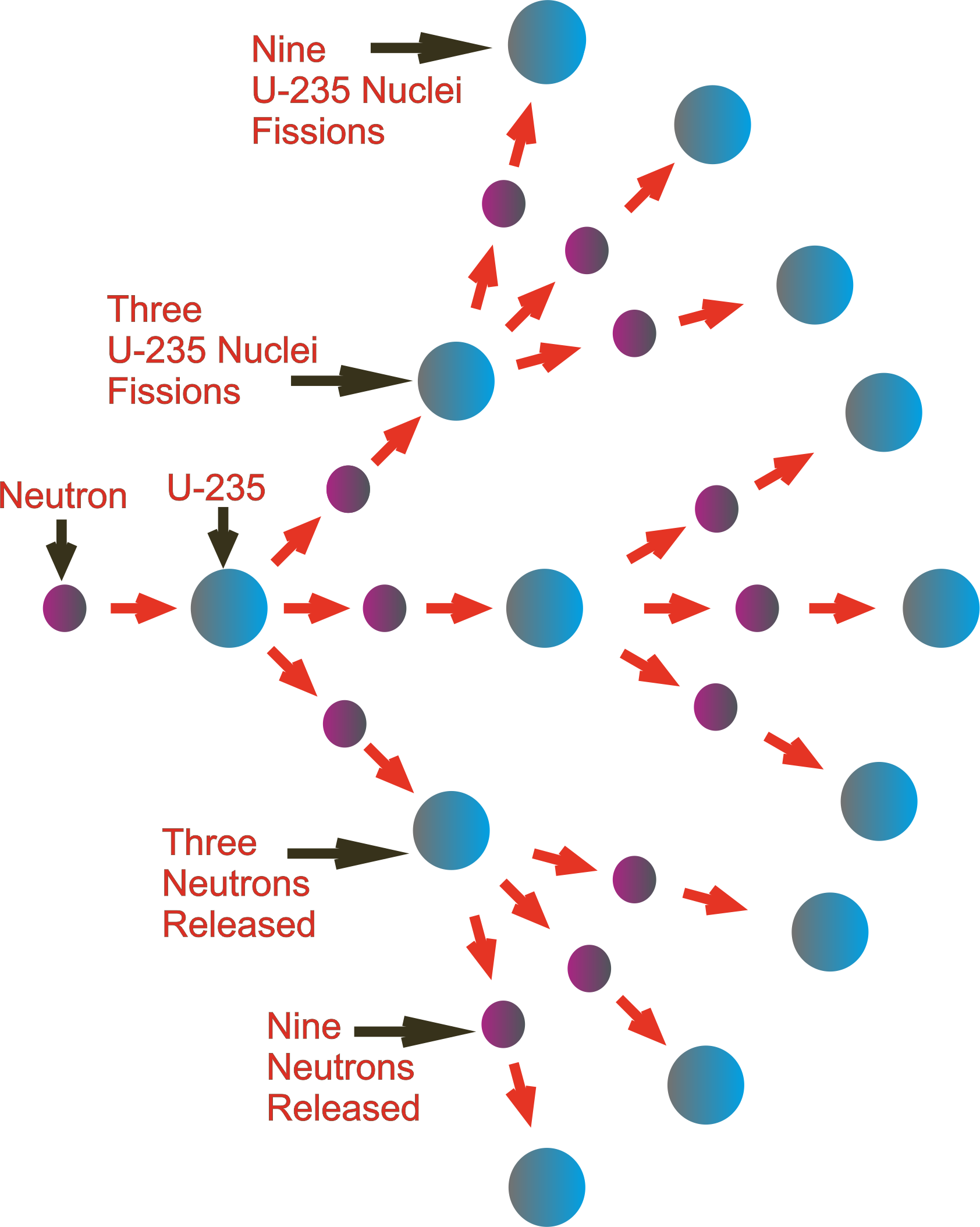
The fission of a single atom of 235U releases 202 million electronvolts (a unit used in electromagnetism and chemistry to measure the work done by an electron to cross a potential difference of 1 volt). It can only be stopped if we find a way to catch more stray neutrons than are formed and prevent them from hitting and breaking other 235U atoms. But that's not all: if there are many other 235U atoms surrounding the one that broke, the three neutrons produced by its fission can hit them and break them each of them will release three neutrons that in turn. The overcoming of the strong fundamental forces holding the two Barium and Krypton nuclei and the three neutrons together also releases a lot of energy in the form of kinetic energy. There are three neutrons missing, which have in fact broken away and shot off as three independent particles. But if we add up their total mass, we see that it comes to 141+92=233. That makes a total of 92 and the proton count adds up. This process is called fission.īarium and Krypton have 36 and 56 protons respectively. It is still Uranium, because we already know that when you change the number of neutrons in an atom you do not change its nature, but the nucleus itself is no longer 235U: it becomes 236U, which reminds us that there is an extra neutron in it.Ģ36U, however, is terribly unstable: as soon as it forms, it falls apart, giving rise to a Barium 141Ba atom and a Krypton 92Kr atom.
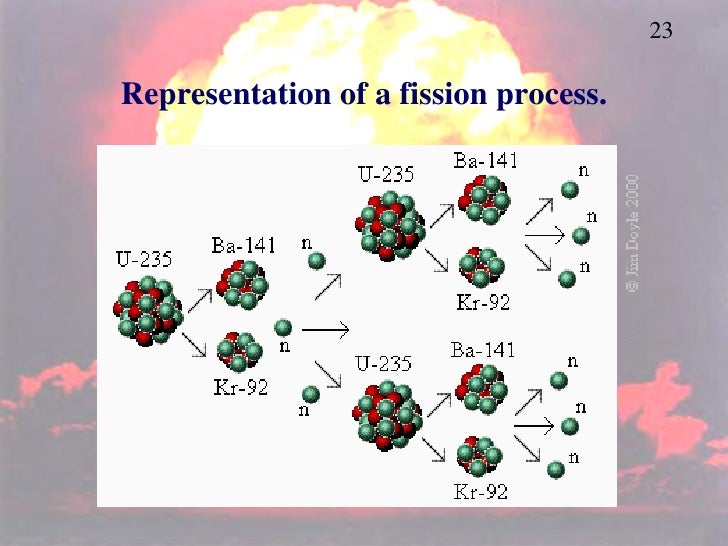
When the 235U atom is hit by a neutron with appropriate kinetic energy, a new isotope is formed. This isotope, remember, has its 92 protons, like all uranium atoms, but has "only" 235-92=143 neutrons. To achieve this, we must choose an isotope of an atom for example, the heaviest available on Earth, 235U. Nuclear fission occurs when a heavy nucleus breaks up and is split into two lighter ones, whose masses (when added together) are less than the original mass. In terms of spelling there is only a different vowel and a consonant between them, but in reality these are two exactly opposite reactions in the atom.


 0 kommentar(er)
0 kommentar(er)
